CAS in Regulatory Affairs
School for Translational Medicine and Biomedical Entrepreneurship (sitem-insel School)
Regulatory specialists are integral to bringing novel medical devices to market. They require a breadth of managerial and interpersonal skills in addition to technical, clinical and legal knowledge. The program offers career specialized training in Regulatory Affairs, Quality Management and Advanced Regulatory Affairs with particular focus on the European Medical Device Regulations. It addresses graduates interested in commencing a career in the regulation or quality control of medical devices and also targets those from complementary disciplines such as entrepreneurship, research and development, and management, seeking comprehensive and practical knowledge in this field.
Boost your skills with our Regulatory Affairs program
| Degree | Certificate of Advanced Studies in Regulatory Affairs, Universität Bern (CAS RA Unibe) |
|---|---|
| Start | 2025 |
| Length | 2 Semester |
| Scope | 15 ECTS |
| Cycle | Annual |
| Flexible entry possible | Yes |
| Single module visitable | Yes |
| Place | sitem-insel, Bern |
| Language | English |
| Admission | Min. Bachelor's degree from a university or university of applied sciences in the fields of natural sciences, engineering, medicine, pharmacy or law. “Sur dossier” admissions possible. |
| Registration until | 2025/07/15 |
| Cost | CHF 12'600 |
| Special Offer | Upon request |
| Organising institutions | School for Translational Medicine and Biomedical Entrepreneurship (sitem-insel School) |
About the program
Regulatory specialists are integral to bringing novel medical devices to market. They require a breadth of managerial and interpersonal skills in addition to technical, clinical and legal knowledge. The program of Advanced Studies in Regulatory Affairs and Quality Management (RAQM) offers comprehensive knowledge and career specialised training for graduate students.
What to expect from this program?
- Increase your carreer prospects
- Obtain an internationally recognised degree from a prestigious Swiss university
- Gain expert insights from academia and industry
- Connect and exchange with peers and lecturers
- Acquire extensive knowledge and skills development in relevant topics
- Keep up with the latest regulatory changes and implementations
- Pursue a "Master of Advanced Studies" title in a stepwise approach
- Profit from small class sizes and flexibility of study formats
Who is the program for?
The program addresses university graduates interested in commencing a career in regulatory affairs or quality management within a medical device company or regulatory body. It also addresses mid-level experienced professionals who would like to raise their proficiency to the next level and approach different career options. Lastly, the program also targets those from complementary disciplines such as entrepreneurship, research and development, and management, seeking comprehensive and practical knowledge of the regulation of medical devices according to the new European MDR or quality management of such products.
Recent graduates will acquire comprehensive knowledge and skill, increase their career projects expand their personal network through close collaboration with peers and lecturers.
Program structure
Design your own study journey
The modular structure of the continuing education program in Regulatory Affairs and Quality Mangement allows you to pursue your goals tailored to your own needs. You can start with a Certificate of Advanced Studies (CAS) and gradually build it up to a Diploma or Master of Advanced Studies in Regulatory Affairs and Quality Management (60 ECTS) degree over time.
Scope
Each CAS corresponds to at least 15 ECTS credits, which are earned by completing at least three modules. One ECTS equals a study work performance of about 25-30 hours, including in-person events, preparatory tasks and all other course activities. The program is conceptualized as an extra-occupational program that can be reconciled with your professional work. The modules are taught in a blended learning environment and utilize e-learning, peer learning and interactive discussions with experts, on site lectures, workshops, and case studies. Small class sizes allow for large flexibility while at the same time permitting to profit from the lecturers’ expertise.
After successful participations, the University of Bern awards the degree Certificate of Advanced Studies in Regulatory Affairs.
Modules
Organising institution and faculty
Swiss Institute for Translational and Entrepreneurial Medicine (sitem-insel)
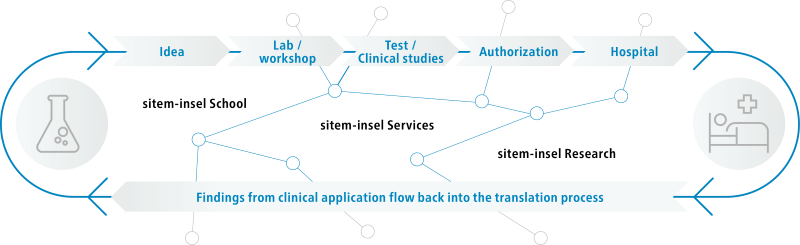
sitem-insel is the Swiss Institute for Translational and Entrepreneurial Medicine. It is located on the Insel Campus Bern and benefits from its proximity to Switzerland's largest university hospital (Inselspital) as well as the University of Bern. At sitem-insel, a wide variety of units from clinics, industry, research, and education are networked under one roof, driving innovation for the benefit of patients. Our mission is to establish, operate and develop a National Center of Excellence for Translational Medicine that professionalizes translational research for the benefit of patients, society, and science. An onsite cutting-edge 20,000 m2 facility is the sitem-insel catalyst for a multidisciplinary collaborative approach to unlocking ‘bench to bedside’ innovation.
Bringing innovation to the patient – by connecting people.
How to get a research idea from bench to bedside?
Together with you, we are committed to bridging the gap between clinical practice, research, entrepreneurship and regulation.
Medical Faculty of the University of Bern
Our education programs in translational medicine, biomedical entrepreneurship, regulatory affairs and artificial intelligence are offered under the umbrella of the Medical Faculty of the University of Bern.
The Medical Faculty is known for an excellent curriculum in human and dental medicine, and also offers an attractive and high-quality range of training and continuing education in the field of health and medicine. With several institutes and clinics in various disciplines, it has excellent, internationally recognized research performance. It also conducts cutting-edge research in artificial intelligence for medicine and provides access to data science for all research groups.
Experts from industry and academia
Our dedicated faculty of senior experts from academia, industry, and regulatory authorities carry the teaching responsibility and support us in educating healthcare and regulatory specialists, bridging knowledge gaps, and advancing professional careers. Observing how our personalized education model fosters innovative and entrepreneurial thinking, we invite you to become part of our network.
Admission, fees and installments
Admission requirements
Applicants must hold at least a Bachelor's degree from a university or university of applied sciences in the fields of natural sciences and engineering, medicine, pharmacy or law. Professional experience is not mandatory. If the applicant has no prior academic degree or professional experience, the study commission may define further conditions for the applicant to successfully complete the course.
Admission process
Please submit your complete application including all required attachments via the registration form. Extensions of application deadlines may be granted by the Directorate.
Once we have received your application, we will gladly evaluate your documents and get back to you within a few working days. Upon successful registration, you will get a Campus Account from the University of Bern, which allows access to the student area of the University websites, the University´s WLAN network (eduroam), the use of library databases and of e-journals. MAS students will also receive a UNICARD and have access to sports, childcare and counselling facilities offered by the University of Bern.
Deadlines
Application for fall term: 15th July
Application for spring term: 15th December
Standalone modules can be admitted anytime, provided places are still available.
The Directorat may grant exceptions on the above deadlines.
Tuition fees & installments
Master of Advanced Studies (MAS): CHF 31'500.–
Diploma of Advanced Studies (DAS): CHF 23'100.–
Certificate of Advanced Studies (CAS): CHF 12'600.–
The full study fee can be paid in full at once or in installments per semester.
There is no entitlement to a refund or waiver of the course fees if parts of the course are not attended. Costs for travel, accommodation and catering are not included. Insurance is in the sole responsibility of participants (accident, travel, cancellation, etc.).
Withdrawal of the registration before the registration deadline is possible without cost consequences. In case of withdrawal after the registration deadline, the full course fee must be paid.
Contact

Dina Marti
Associate Courses
CAS in Artificial Intelligence in Medical Imaging
| Degree | CAS |
|---|---|
| Start | 2025 |
| Language | Englisch |
| Cost | CHF 12'600.- |
The program equips medical professionals, as well as scientists from the fields of medicine, pharmacy, natural sciences and technology (engineering, computer science, etc.) with the necessary knowledge and skills to translate medical problems to data science problems and hence to actively engage in the environment of digital healthcare. Participants will learn about the basic concepts of artificial intelligence (AI) algorithms, their applications and their integration into the clinical work process.
CAS in Translational Medicine
| Degree | CAS |
|---|---|
| Start | 2025 |
| Language | Englisch |
| Cost | CHF 12'600 |
The program includes all relevant aspects of the translational process, including basic scientific, clinical, and technical knowledge. It covers different points relevant for the development and commercialization of medicinal products and medical devices.
DAS in Translational Medicine
| Degree | DAS |
|---|---|
| Start | 2025 |
| Language | Englisch |
| Cost | CHF 23'100 |
The program includes all relevant aspects of the translational process – entrepreneurial know-how in addition to all basic scientific, clinical, and technical knowledge. It covers different points relevant for the development and commercialization of medicinal products and medical devices.
MAS in Translational Medicine and Biomedical Entrepreneurship
| Degree | MAS |
|---|---|
| Start | 2025 |
| Language | Englisch |
| Cost | CHF 31'500 |
The program includes all relevant aspects of the translational process – entrepreneurial know-how in addition to all basic scientific, clinical, and technical knowledge. It covers different points relevant for the development and commercialization of medicinal products and medical devices.